Soil nutrients that are available to your plants are greatly affected by soil pH. If there is an “ideal” soil pH, it is close to neutral meaning the soil falls within a range from a slightly acidic pH of 6.5 to slightly alkaline pH of 7.5. It has been determined that most plant nutrients are optimally available to plants within this 6.5 to 7.5 pH range, plus this range of pH is generally very compatible to plant root growth. [1]
Any plant suffering from a deficiency of lime (i.e. growing in a pH group that is too low) will show stunting symptoms and the growing tips of the plant are yellow and deformed while the lower parts of the plant remain unaffected. This is often accompanied by short and stubby root growth instead of long fibrous ones.
At very low pH levels, aluminum and manganese are dissolved by acids and escape into the soil. These are poisonous to some plants.
On the other hand, plants growing in a lime soil (where the pH is too high) can also be adversely affected. This is generally more because of a secondary effect on other plant foods than because of an excess of Calcium.
Symptoms of high pH levels in soil usually show as deficiencies of Iron, Boron, or Manganese. Unfortunately, these also result in yellowing of the leaves which makes detection a bit more difficult.


With Iron deficiency, the leaf veins usually remain a deep green.
Boron deficiency shows up as twisted, distorted growth and often the terminal bud dies. In turnip and beetroot, hollow, brown areas develop.

With a Manganese deficiency the terminal bud stays alive, but the older leaves show yellow patches between the veins, and often dead spots appear on the leaves.

If these deficiencies are present because your soil has too much lime present, they cannot be corrected by applying the appropriate element (i.e. and acid), because no matter how much is applied, the presence of the excess lime in the soil “locks up” the elements and makes them unavailable to plant roots. The way to unlock them is to modify the pH level. This frees the elements in the soil eliminating the need to add “extra” amounts of those that were deficient.
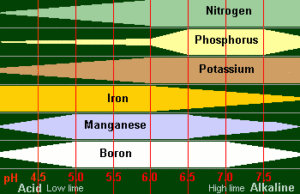
The chart shows this quite clearly. You can see how the main plant foods of Nitrogen, Phosphate and Potash are made more or less available at different pH levels. The availability of Iron, Manganese and Boron is also depicted.
From the chart you can see that it is pointless to apply Phosphate fertilizers if your pH is very low, because it would not be available.
By increasing your pH to around 6.5, more Phosphate is available (FREE!). This is OK provided that the plants you want to grow are happy at pH 6.5 (see lists). You will, no doubt have noticed that the majority of plant foods are at their most available at pH 6.5 which may be why it is often quoted as being the best general pH level for most soils.
An application of lime also has other advantages provided that it does not raise your pH to a level that is higher than that needed for the sort of plants you want to grow.
Lime helps to improve soil drainage, aeration and work-ability of clay soils by making them less sticky and more open. It does this by creating sand sized multi-particles. It encourages worm activity which itself significantly increases the organic content of the soil as “food”pulled into the soil by worms decomposes. It also helps to prevent some diseases, e.g. Club Root of Brassicas.
See also…
Identifying Your Soil Type
How pH Affects Plant Foods
Finding Soil’s pH
Raising Soil pH
Lowering Soil pH
References:
- Soil pH and the Availability of Plant Nutrients Retrieved Dec 6, 2010 from Jensen, Dr. Thomas L., “Soil pH and the Availability of Plant Nutrients,” IPNI Plant Nutrition TODAY, Fall 2010, No. 2, www.ipni.net/pnt



this is a great article. i am sorry if i missed the information. but copied the paragraph below for a reference. you say to unlock the elements you must modify the ph level, but you say you can’t just add another element to do that. so my question becomes. well how do i go about modifying the ph level then?????
If these deficiencies are induced because your soil has too much lime present, they cannot be corrected by applying the appropriate element, because no matter how much is applied, the presence of the excess lime in the soil “locks up” the elements and makes them unavailable to plant roots. The way to unlock them is to modify the pH level. This frees the elements in the soil eliminating the need to add “extra” amounts of those that were deficient.
Janet, sorry for the late reply… I would direct you to this article…
https://farmhomestead.com/identify-soil-type/
at the bottom there are more links to walk you through this. After reading it, if it doesn’t answer your question, then please let me know and I will elaborate. 🙂
YoYo